MEDIUM
NEET
IMPORTANT
Earn 100
The standard reduction potentials at of and are and
respectively. Which one of the following is the strongest oxidising agent?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Electrochemistry
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Given : and . Which of the following statements is correct?
HARD
NEET
IMPORTANT
Consider the following sets:
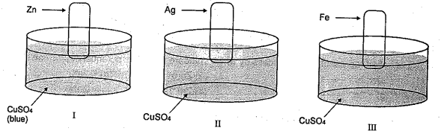
Blue colour solution changes to colourless (or fades) in :
HARD
NEET
IMPORTANT
Consider the following sets
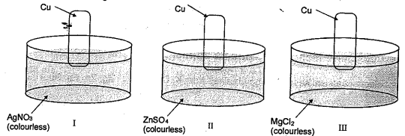
colourless solution changes to blue coloured solution in:
HARD
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
