HARD
11th CBSE
IMPORTANT
Earn 100
The standard state Gibbs free energies of formation of are
The standard state means that the pressure should be 1 bar, and substance should be pure at a given temperature. The conversion of graphite to diamond reduces its volume by . If graphite is converted to diamond isothermally at , the pressure at which graphite is in equilibrium with diamond is
[Useful information :, , ]
(a)
(b)
(c)
(d)
40% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
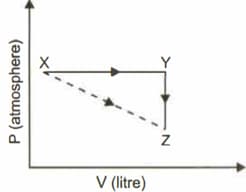
For an ideal gas, consider only work in going from an initial state to the final state . The final state can be reached by either of the two paths shown in the figure. Which of the following choice(s) is (are) correct ? [take as change in entropy and as work done].