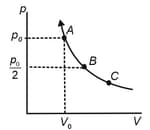
The state of an ideal gas is changed through an isothermal process at the temperature as shown in the figure. The work done by the gas in going from state to is double the work done by the gas in going from state to . If the pressure in the state is , then the pressure of the gas in the state is


Important Questions on Laws of Thermodynamics
diagram of two moles of a mono-atomic gas is as shown in the figure. For the process, choose the correct options given below
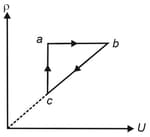
The density versus internal energy Graph of gas is as shown in the figure. Choose the correct options.
Here, is work done by the gas and is the heat given to the gas.
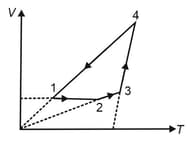
A cyclic process is depicted on diagram. The and diagrams for this cyclic process are given below. Select the correct choices (more than one options is/are correct)
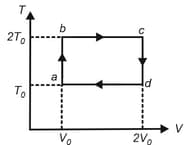
One mole of a monatomic ideal gas is taken along the cycle as shown in the diagram.
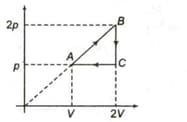
The ratio of specific heat in the process to the specific heat in the process is
One mole of a monoatomic ideal gas is taken through the cycle as shown in the figure.
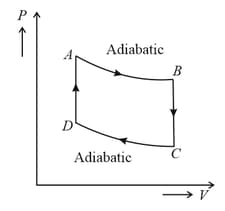
The temperature at is