EASY
NEET
IMPORTANT
Earn 100
The strength of bonds by overlap is generally in the order:
(a)
(b)
(c)
(d)
35% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
NEET
IMPORTANT
Which of the following -orbitals overlapping would result in the strongest bond:
EASY
NEET
IMPORTANT
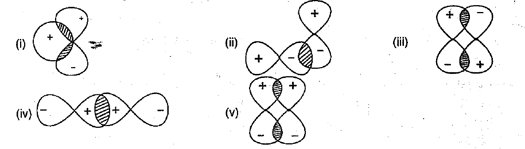
Which of the following atomic orbital overlapping are not allowed:
EASY
NEET
IMPORTANT
Indicate the correct statement according to VBT:
EASY
NEET
IMPORTANT
Which of the following overlapping is correct [assuming X-axis to be the internuclear axis]:
EASY
NEET
IMPORTANT
Which statement is correct?
EASY
NEET
IMPORTANT
Total no of bonds are present in respectively.
EASY
NEET
IMPORTANT
Which is not same for the resonating structures?
EASY
NEET
IMPORTANT
The correct representation of Lewis dot structure of
