The structural formula of an ester is shown below:
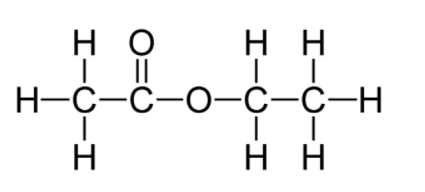
Write the formula of the acid and the alcohol from which it is formed.


Important Questions on Carbon And its Compounds
Consider the following organic compounds:
Out of these compounds, which compound is most likely to be sweet-smelling?
Consider the following organic compounds:
Out of these compounds, which compound on treatment with concentrated at forms an alkene?
Consider the following organic compounds:
Out of these compounds, which compound on repeated chlorination forms chloroform?
Consider the following organic compounds:
Out of these compounds, which compound is added to alcohol to denature it?
Consider the following organic compounds:
Out of these compounds, which compound is a constituent of vinegar?
Consider the following organic compounds:
Out of these compounds, which compound is used to sterilise wounds and syringes?
An organic acid is a liquid, which often freezes during winter time in cold countries, having the molecular formula . On warming it with methanol in the presence of a few drops of concentrated sulphuric acid, a compound with a sweet smell is formed.
Identify and . Also write their formulae showing the functional group present in them.
An organic acid is a liquid, which often freezes during winter time in cold countries, having the molecular formula . On warming it with methanol in the presence of a few drops of concentrated sulphuric acid, a compound with a sweet smell is formed.
Write a chemical equation for the reaction involved.
