MEDIUM
JEE Main
IMPORTANT
Earn 100
The structures of and formed in the following reaction are :
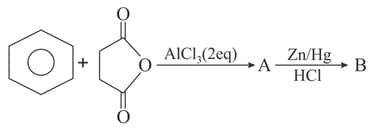
(a)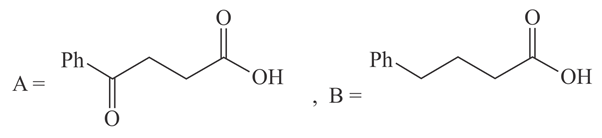
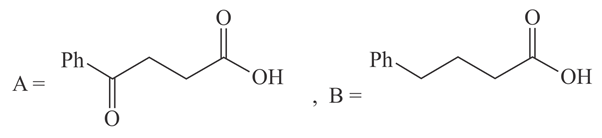
(b)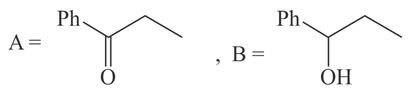
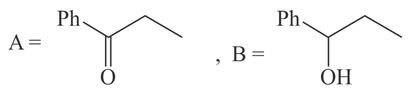
(c)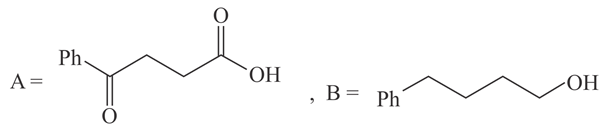
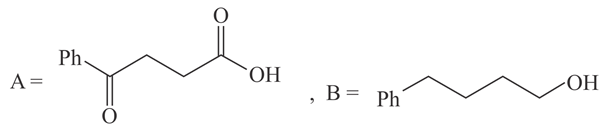
(d)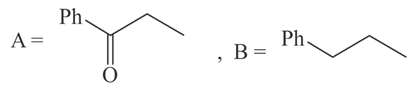
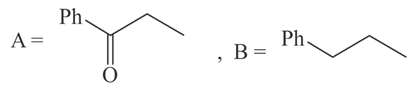
50% studentsanswered this correctly

Important Questions on Hydrocarbons
MEDIUM
JEE Main
IMPORTANT
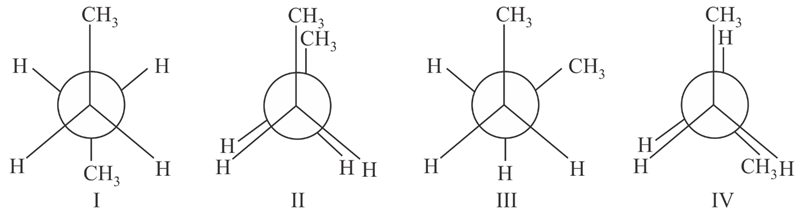
Arrange the following conformational isomers of n-butane in order of their increasing potential energy:
EASY
JEE Main
IMPORTANT
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): Treatment of bromine water with propene yields 1-bromopropan-2-ol.
Reason (R) : Attack of water on bromonium ion follows Markovnikov rule and results in 1-bromopropan-2-ol.
In the light of the above statements, choose the most appropriate answer from the options given below :
MEDIUM
JEE Main
IMPORTANT
The structure of product formed by the following sequence of reactions is :
MEDIUM
JEE Main
IMPORTANT
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : Metallic character decreases and non-metallic character increases on moving from left to right in a period.
Reason (R): It is due to increase in ionisation enthalpy and decrease in electron gain enthalpy, when one moves from left to right in a period.
In the light of the above statements, choose the most appropriate answer from the options given below :
HARD
JEE Main
IMPORTANT
The total number of monohalogenated organic products in the following (including stereoisomers) reaction is
MEDIUM
JEE Main
IMPORTANT
Methylpentene on reaction with in presence of peroxide forms an addition product. The number of possible stereoisomers for the product is
EASY
JEE Main
IMPORTANT
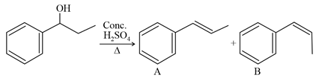
consider the above reaction, and choose the correct statement:
EASY
JEE Main
IMPORTANT
The dihedral angle in staggered form of Newmann's projection of-Trichloro ethane is degree. (Round off the answer to the nearest integer)
