HARD
Earn 100
The study of emission or absorption spectra is referred to as
(a)spectroscopy
(b)atomic spectra
(c)line spectra
(d)continuous spectrum
76.67% studentsanswered this correctly
Important Questions on Structure of Atom
EASY
MEDIUM
EASY
The number of following statement/s which is/are incorrect is ______
A) Line emission spectra are used to study the electronic structure
B) The emission spectra of atoms in the gas phase show a continuous spread of wavelength from red to violet.
C) An absorption spectrum is like the photographic negative of an emission spectrum
D) The element helium was discovered in the sun by spectroscopic method
MEDIUM
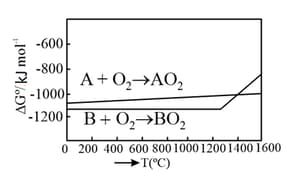
MEDIUM
HARD
.
This equation reduces to the perfect gas equation, When,
MEDIUM
(Avogadro constant )
MEDIUM
MEDIUM
MEDIUM
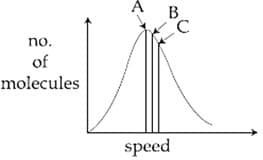
Root mean square speed most proable speed Average speed
HARD
MEDIUM
A solution containing impure reacts completely with of in acid solution. The purity of (in ) is______ (mol. wt. of ; mol. wt. of )
MEDIUM
EASY
MEDIUM
MEDIUM
A solution was made by adding of The normality of the solution is . The value of is _______
(The atomic mass of is )
EASY
Which one of the following has the highest wavelength?
EASY
(A) Hydrogen has one electron in its orbit but it produces several spectral lines.
(R) There are many excited energy levels available.
MEDIUM

