MEDIUM
JEE Main
IMPORTANT
Earn 100
The successive ionisation energies in of an element are and . Which ion is the most likely to be formed when reacts with chloride?
(a)
(b)
(c)
(d)
62.56% studentsanswered this correctly

Important Questions on Classification of Elements and Periodicity in Properties
EASY
JEE Main
IMPORTANT
The and of an element are , respectively. The element is likely to be:
EASY
JEE Main
IMPORTANT
Successive ionisation energies of an element are given below (in ):
Electronic configuration of the element is:
EASY
JEE Main
IMPORTANT
Which systematic diagram is correct about the ionisation energy of coinage metals?
EASY
JEE Main
IMPORTANT
Which of following represents the correct order of ?
MEDIUM
JEE Main
IMPORTANT
How many of the following have greater than Silicon atom:
(i) (ii) (iii) (iv) (v) (vi) (vii) (viii) (ix)
MEDIUM
JEE Main
IMPORTANT
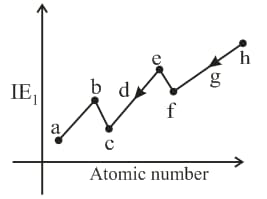
Where are period elements. If the difference between the atomic numbers of elements and is and the difference between the atomic number of elements and is What is the value of
MEDIUM
JEE Main
IMPORTANT
The electron gain enthalpy of a hypothetical element is per atom. How much energy in is released when of are completely converted to , ions in gaseous state ? (Molar mass of )
EASY
JEE Main
IMPORTANT
Find the total number of cations for which of cation is lower than corresponding atom.
