EASY
JEE Main
IMPORTANT
Earn 100
The temperature of a substance increases by . On the Kelvin scale, this increase is equal to
100% studentsanswered this correctly

Important Questions on Thermometry, Thermal Expansion And Calorimetry
EASY
JEE Main
IMPORTANT
Maximum density of is at a temperature of (in Fahrenheit scale) _____ .
EASY
JEE Main
IMPORTANT
The temperature of a body on Kelvin scale is found to be . When it is measured by Fahrenheit thermometer, it is found to be . The value of is _____.
EASY
JEE Main
IMPORTANT
A centigrade and a Fahrenheit thermometers are dipped in boiling water. The temperature of water is lowered until the Fahrenheit thermometer registers . The temperature as registered by the centigrade thermometer is _____.
EASY
JEE Main
IMPORTANT
The centigrade (Celsius) and Fahrenheit readings are same at _____.
EASY
JEE Main
IMPORTANT
On centigrade scale, the temperature of a body increases by . The increase in temperature on Fahrenheit scale is _____.
MEDIUM
JEE Main
IMPORTANT
Four cubes of ice at , each , are taken out from the refrigerator and put in of water at . Find the temperature of water when thermal equilibrium is attained. Assume that no heat is lost to the outside and the water equivalent of the container is . (Specific heat capacity of water , specific heat capacity of ice , latent heat of fusion of ice .)
MEDIUM
JEE Main
IMPORTANT
An ice block at and mass is dropped from a height such that the loss in gravitational potential energy of the block is exactly equal to the heat required to just completely melt the ice. Taking latent heat of fusion of ice as , acceleration due to gravity as and mechanical equivalent of heat as , find the value of .
MEDIUM
JEE Main
IMPORTANT
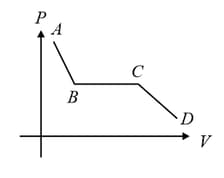
The portion of the indicator diagram representing the state of matter denotes: