The tenth elements in the periodic table resemble with the
Important Questions on Classification of Elements and Periodicity in Properties
Match the element in column I with that in column II.
| Column-I | Column-II | ||
| (a) | Copper | (p) | Non-metal |
| (b) | Fluorine | (q) | Transition metal |
| (c) | Silicon | (r) | Lanthanoid |
| (d) | Cerium | (s) | Metalloid |
Identify the correct match :
The correct match among the following is
(a) Lithium, Sodium, Potassium (i) Alkaline earth metals
(b) Beryllium, Magnesium, Calcium (ii) Semi-metals
(c) Oxygen, Sulphur, Selenium (iii) Alkali metaIs
(d) Silicon, Germanium, Arsenic (iv) Chalcogens
(atomic number: , ,
Assertion: For hydrogenation reactions, the catalytic activity increases from Group to Group metals with maximum activity shown by Group elements.
Reason: The reactants are most strongly adsorbed on group elements.
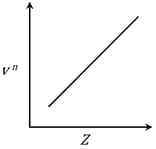
Given Frequency of X-ray emitted
Atomic number
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power 'n' i.e. of X-rays emitted is plotted against atomic number , the following graph is obtained.
The value of 'n' is
Consider the following radioactive decays
I. and
II.
In which case group of parent and daughter elements remains unchanged.
Match List - I with List - II
| LIST-I (Atomic number) |
LIST-II (Block of periodic table) |
||
| (A) | I. | -block | |
| (B) | II. | -block | |
| (C) | III. | -block | |
| (D) | IV. | -block | |
Choose the correct answer from the options given below: