EASY
Earn 100
The titration curve for titration of a solution of a diprotic acid with is shown below.
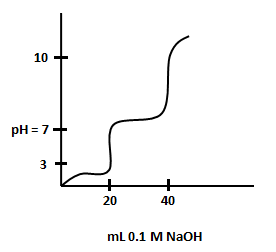
and are approximately

(a)
(b)
(c)
(d)
100% studentsanswered this correctly
Important Questions on Equilibria
EASY
EASY
EASY
MEDIUM
MEDIUM
Statement I : In the titration between strong acid and weak base methyl orange is suitable as an indicator.
Statement II : For titration of acetic acid with $\mathrm{NaOH}$ phenolphthalein is not a suitable indicator.
In the light of the above statements, choose the most appropriate answer from the options given below:
HARD
The pH of the solution after Expt. 2 is
HARD
MEDIUM
Find the nonnality of solution, if of it is required to react completely with of solution. ( Molar mass of )
HARD
HARD
MEDIUM
HARD
HARD
A solution of weak base is titrated with of a strong acid . The variation of of the solution with the volume of added is shown in the figure below. What is the of the base? The neutralisation reaction is given by .
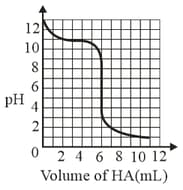
EASY
HARD
EASY
MEDIUM
HARD
MEDIUM
MEDIUM

