MEDIUM
Earn 100
The total number of elements of symmetry in a cubic crystal is
(a)
(b)
(c)
(d)none of these
76.92% studentsanswered this correctly
Important Questions on Solid State
HARD
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
HARD
EASY
HARD
EASY
HARD
MEDIUM
HARD
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).
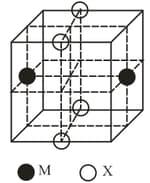
MEDIUM
EASY

