HARD
NEET
IMPORTANT
Earn 100
The total volume of gases obtained at by electrolysis of aqueous sodium acetate, by passing current for minutes, is
(a) litres
(b) litres
(c) litres
(d) litres
50% studentsanswered this correctly

Important Questions on Electrochemistry
HARD
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
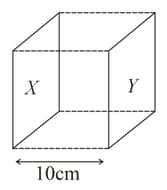
The conductance of a salt solution () measured by two parallel electrodes of the below cube was found to be If the volume enclosed between two electrodes contains moles of salt, what is the molar conductivity of the salt at the same concentration?
HARD
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
The molar conductivity of methanoic acid is Then, its degree of dissociation is
[Given: and
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
