EASY
Earn 100
The type of absorption is highly specific and takes place at high temperature with unimolecular layer is
(a)Chemisorption
(b)Physisorption
(c)Chemisorption and physisorption
(d)None of the above
50% studentsanswered this correctly
Important Questions on Surface Chemistry
MEDIUM
MEDIUM
MEDIUM
EASY
Given below are the critical temperatures of some of the gases:
| Gas | Critical temperature |
The gas showing least adsorption on a definite amount of charcoal is
EASY
MEDIUM
MEDIUM
EASY
( is the mass of the gas adsorbed per gram of adsorbent)
MEDIUM
EASY
EASY
HARD
EASY
Match List I with List II
| List I | List II | ||
| A | Physisorption | I | Single Layer Adsorption |
| B | Chemisorption | II | |
| C | III | Chromatography | |
| D | Analytical Application or Adsorption | IV | Heterogeneous catalysis |
Choose the correct answer from the options given below:
HARD
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
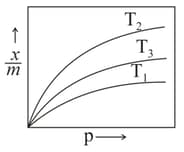
The correct order of the temperatures is:

