EASY
JEE Main
IMPORTANT
Earn 100
The unit of the van der Waals gas equation parameter in is:
50% studentsanswered this correctly
Important Questions on States of Matter: Gases and Liquids
EASY
JEE Main
IMPORTANT
A certain gas obeys . The value of is . The value of is (Integer answer) ( : compressibility factor)
EASY
JEE Main
IMPORTANT
The interaction energy of London forces between two particles is proportional to , where is the distance between the particles. The value of is :
EASY
JEE Main
IMPORTANT
An LPG cylinder contains gas at a pressure of at . The cylinder can withstand the pressure of . The room in which the cylinder is kept catches fire. The minimum temperature at which the bursting of cylinder will take place is __________ . (Nearest integer)
EASY
JEE Main
IMPORTANT
A home owner uses of methane gas, (assume is an ideal gas) in a year to heat his home. Under the pressure of atm and , mass of gas used is . The value of is ______. (Nearest integer)
(Given )
MEDIUM
JEE Main
IMPORTANT
A car tyre is filled with nitrogen gas at psi at . It will burst if pressure exceeds psi. The temperature in at which the car tyre will burst is (Rounded-off to the nearest integer)
EASY
JEE Main
IMPORTANT
The volume occupied by of acetylene gas at and pressure is ____________ (Rounded off to the nearest integer)
[Given ]
MEDIUM
JEE Main
IMPORTANT
Match the type of interaction in column with the distance dependence of their interaction energy in column
| A | B |
| (i) ion - ion | (a) |
| (ii) Dipole - dipole | (b) |
| (iii) London dispersion | (c) |
| (d) |
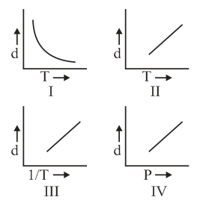
HARD
JEE Main
IMPORTANT
Which one of the following graphs is not correct for ideal gas?
Density, Pressure, Temperature