HARD
JEE Main/Advance
IMPORTANT
Earn 100
The value of of (graphite) (diamond) is at entropy of graphite is higher than entropy of diamond. This implies that:
(a) (diamond) is more thermodynamically stable than (graphite) at
(b) (graphite) is more thermodynamically stable than (diamond) at
(c)diamond will provide more heat on complete combustion at
(d) (diamond) (graphite) is
50% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
Compute the temperature above, which the given reaction will be spontaneous.
HARD
JEE Main/Advance
IMPORTANT
Select the correct molar entropy at corresponding temperature using following data:
(i) Heat capacity of solid from to normal melting point
(ii) Enthalpy of fusion
(iii) Enthalpy of vaporisation
(iv) Heat capacity of liquid form to normal boiling point
(v) Heat capacity of gas from to at atm
EASY
JEE Main/Advance
IMPORTANT
Select correct statement?
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
Hydrazine used in rocket fuels can be obtained by the reaction of ammonia and hydrogen peroxide according to the following equations
If (formation) of and are and respectively, for the decomposition of hydrazine into and is
MEDIUM
JEE Main/Advance
IMPORTANT
One mole of Ideal gas . follow the process as shown in figure. Predict the following:
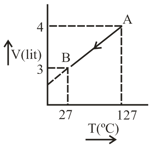
Calculate work done, Heat of process.
