EASY
MHT-CET
IMPORTANT
Earn 100
The values of potential energy, kinetic energy and the total energy of the electron in the fourth orbit of hydrogen atom are respectively___ (Energy of electron in the ground state is )
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Atoms, Molecules and Nuclei
EASY
MHT-CET
IMPORTANT
Total energy of electron in an excited state of hydrogen atom is . The kinetic and potential energy of electron in this state
EASY
MHT-CET
IMPORTANT
The graph between wave number and angular frequency () is
EASY
MHT-CET
IMPORTANT
The transition of an electron from , to gives rise to
EASY
MHT-CET
IMPORTANT
The lines of Lyman series are present in which region of the spectrum?
EASY
MHT-CET
IMPORTANT
If and are the wavelength of the first numbers of the Lyman and Paschen series, respectively. Then
EASY
MHT-CET
IMPORTANT
The least energetic wave number in the Paschen series is
EASY
MHT-CET
IMPORTANT
Out of the following which one is not a possible energy for a photon to be emitted by hydrogen atom according to Bohr's atomic model?
EASY
MHT-CET
IMPORTANT
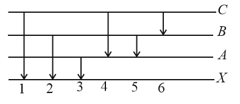
The figure indicates the energy level diagram of an atom and the origin of six spectral lines in emission (for example, line number arises from the transition from level ). Which of the following spectral lines will also occur in the absorption spectrum?