EASY
JEE Main/Advance
IMPORTANT
Earn 100
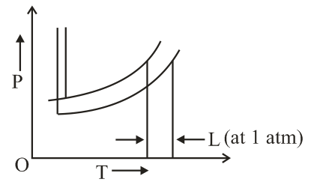
The vapor pressure of chlorobenzene and water at different temperatures are
At what temperature will steam-distillation under a total pressure of ?
(a)
(b)
(c)
(d)
75% studentsanswered this correctly

Important Questions on Solutions
HARD
JEE Main/Advance
IMPORTANT
The vapour pressure of fluorobenzene at is given by the equation
Calculate the boiling point of the liquid in if the external (applied) pressure is more than required for normal boiling point.
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
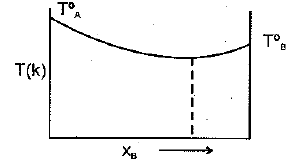
The diagram given below represents boiling point composition diagram of solution of components and which is/are incorrect among the following?
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below: The quantity indicated by in the figure is: