EASY
JEE Main/Advance
IMPORTANT
Earn 100
The velocity of an electron in single electron atom in an orbit
(a)is independent of the atomic number of the element
(b)increases with increase in atomic number
(c)decreases with increase in atomic number
(d)increases with increase in quantum number
50% studentsanswered this correctly

Important Questions on Atomic Physics
EASY
JEE Main/Advance
IMPORTANT
The energy level diagram is for a hypothetical atom. A gas of these atoms initially in the ground state is irradiated with photons having a continuous range of energies between and electron volts. One would) expect photons of which of the following energies to be emitted from the gas?

MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
The diagram to the right shows the lowest four energy levels for an electron in a hypothetical atom. The electron is excited to the level of the atom and transitions to the lowest energy state by emitting only two photons. Which of the following energies could not belong to either of the photons?
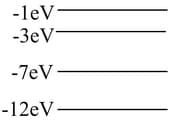
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
