The volume vs. temperature graph of 1 mole of an ideal gas is given below
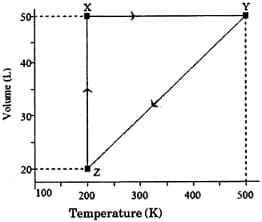
The pressure of the gas (in atm) at and respectively, are
Important Questions on Thermodynamics
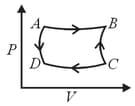
and are isothermal processes while and are adiabatic processes. The same cycle in the temperature - entropy plane is :
At of iron reacts with to form . The evolved hydrogen gas expands against a constant pressure of . The work done by the gas during this expansion is -_______ .
(Round off to the Nearest Integer)
[Given : . Assume, hydrogen is an ideal gas]
[Atomic mass off Fe is ]
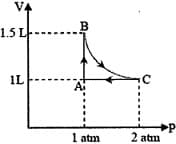
The magnitude of work done by a gas that undergoes a reversible expansion along the path shown in the figure is _________.
Under the isothermal condition, a gas at expands from to against a constant external pressure of bar. The work done by the gas is
(Given that bar)
(Round off to the Nearest Integer)
[Use :
[Assume volume of is much smaller than volume of . Assume treated as an ideal gas]
[ Use ]
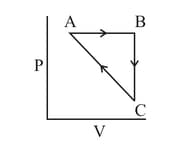
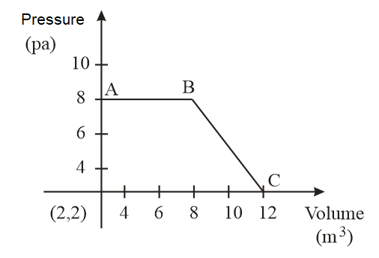
Heat absorbed by the system during process is
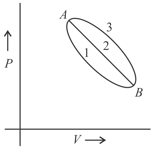
A certain mass of a gas was brought from state to by following three different paths, namely and , respectively. Which of the following relations is correct for the work done?
The combination of plots which does not represent isothermal expansion of an ideal gas is





