EASY
Earn 100
The work done by gravity is given by the formula, . The negative sign shows that the particle is dropping from a height
in the direction of gravity.
50% studentsanswered this correctly
Important Questions on Chemical thermodynamics and energetics
EASY
HARD
MEDIUM
HARD
EASY
MEDIUM

HARD
Match the following
| (A) | Isothermal process | (i) | |
| (B) | Adiabatic process | (ii) | |
| (C) | Isobaric process | (iii) | |
| (D) | Isochoric process | (iv) |
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
MEDIUM
EASY
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is




EASY
HARD

The correct option(s) is (are)
EASY
(Latent heat of ice is and )
HARD

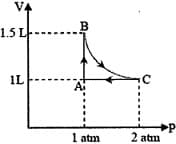
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
EASY
MEDIUM
EASY
EASY
MEDIUM

