EASY
Earn 100
The zinc oxide formed after the process of calcination or roasting undergoes reduction to obtain or extract zinc.
(a)True
(b)False
100% studentsanswered this correctly
Important Questions on Metals and Non-metals
MEDIUM
Which of the following statements are correct for the above chemical reaction?
(i) Lead is reduced.
(ii) Carbon di oxide is oxidised.
(iii) Carbon is oxidised.
(iv) Lead oxide is reduced
MEDIUM
( are the atomic masses)
How much of Iron, we can get if 54 kg of Aluminium is used?
MEDIUM
HARD
HARD
Choose the correct statements about the given chemical reaction:
a) Reaction is exothermic.
b) Al is acting as a reducing agent.
c) is getting reduced.
d) Al is more reactive than Mn
HARD
HARD
Write short note on electrolytic refining of metals.
MEDIUM
EASY
MEDIUM
MEDIUM
Write the steps involved in the extraction of pure metals in the middle of the activity series from their carbonate ores.
EASY
MEDIUM
EASY
HARD
In the given figure, the purification of copper in electric decomposition is shown. Label against and .
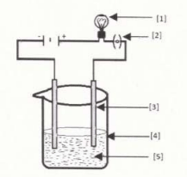
EASY
MEDIUM
EASY

