EASY
Earn 100
There is bonding between two atoms where one atom is having one vacant orbital and another is having one lone pair of electrons. The bond is known as .
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
MEDIUM
EASY
EASY
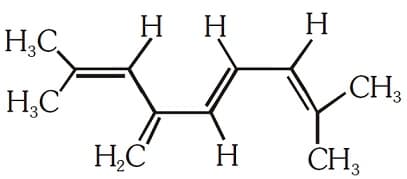
EASY
The total number of and bonds present in the following compound are
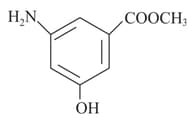
MEDIUM
MEDIUM
Given below are two statements.
Statement I: The presence of weaker -bonds make alkenes less stable than alkanes
Statement II: The strength of the double bond is greater than that of carbon-carbon single bond.
In the light of the above statements, choose the correct answer from the options : given below.
HARD
EASY
EASY
MEDIUM
EASY
EASY
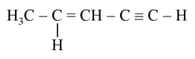 is _______.
is _______.MEDIUM
EASY
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
MEDIUM
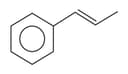
EASY
MEDIUM
MEDIUM
Which one doesn't have bond?

