EASY
NEET
IMPORTANT
Earn 100
Thermodynamic processes are indicated in the following diagram.
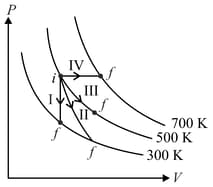
Match the following
Column – 1
Column - 2
P. Process I
a. Adiabatic
Q. Process II
b. Isobaric
R. Process III
c. Isochoric
S. Process IV
d. Isothermal

Match the following
(a)
(b)
(c)
(d)
56.25% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
NEET
IMPORTANT
A gas mixture consists of moles of and 4 moles of at temperature . Neglecting all vibrational modes, the total internal energy of the system is ( is universal gas constant)
EASY
NEET
IMPORTANT
A Carnot's engine having an efficiency of as heat engine, is used as a refrigerator. If the work done on the system is the amount of energy absorbed from the reservoir at lower temperature is
MEDIUM
NEET
IMPORTANT
One mole of an ideal monatomic gas undergoes a process described by the equation constant. The heat capacity of the gas during this process is
EASY
NEET
IMPORTANT
The temperature inside a refrigerator is and the room temperature is . The amount of heat delivered to the room for each joule of electrical energy consumed ideally will be
EASY
NEET
IMPORTANT
A refrigerator works between and . It is required to remove calories of heat every second in order to keep the temperature of the refrigerated space constant. The power required is:
(Take cal = Joules)
EASY
NEET
IMPORTANT
A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half. Then:
EASY
NEET
IMPORTANT
Figure below shows two paths that may be taken by a gas to go from a state A to a state C.
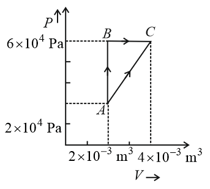
In process AB, of heat is added to the system and in process BC, of heat is added to the system. The heat absorbed by the system in the process AC will be:
EASY
NEET
IMPORTANT
A Carnot engine, having an efficiency of as heat engine, is used as a refrigerator. If the work done on the system is , the amount of energy absorbed from the reservoir at a lower temperature is:
