EASY
Earn 100
Thermodynamics is concerned with:
(a)Kinetics of a change
(b)Energy changes in a system
(c)Rate of chemical change
(d)Mass changes in nuclear reactions
100% studentsanswered this correctly
Important Questions on Chemical thermodynamics and energetics
EASY
Among the following the number of state variable is
Internal energy
Volume
Heat
Enthalpy
EASY
MEDIUM
MEDIUM
EASY
EASY
(Latent heat of ice is and )
EASY
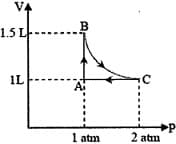
HARD
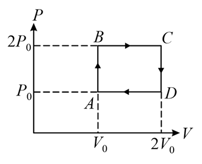
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
MEDIUM
EASY
HARD
EASY
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is
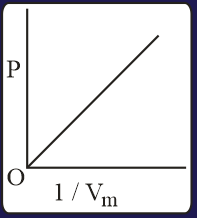
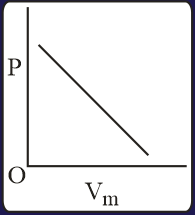
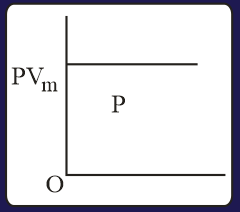
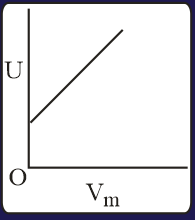
MEDIUM
The total number of intensive properties from the following is _____.
Volume, Molar heat capacity, molarity, ,Gibbs free energy change, Molar mass, Mole
HARD
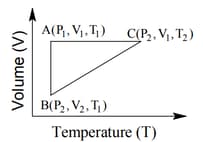
The correct option(s) is (are)
MEDIUM
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
EASY
EASY
EASY
i)
ii)
iii)
iv)

