This diagram shows the electrolysis of copper chloride.
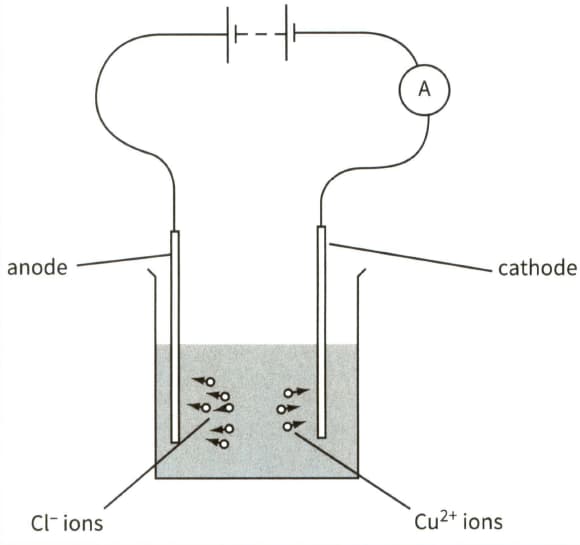
On a copy of the diagram, mark the direction of the conventional current in the electrolyte. Label it conventional current.


Important Questions on Electric Current, Potential Difference and Resistance
This diagram shows the electrolysis of copper chloride.
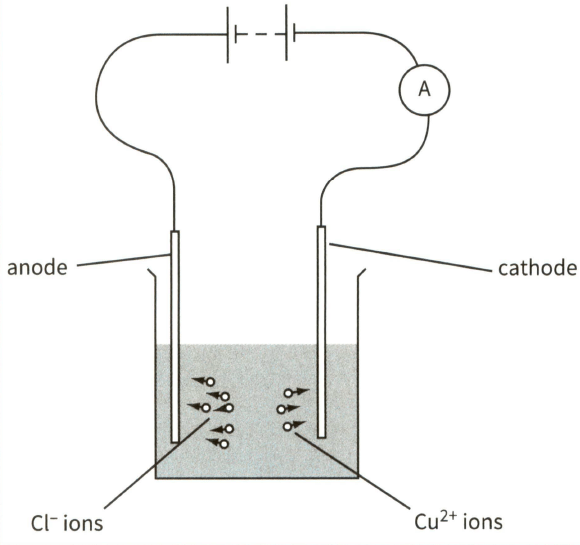
Mark the direction of the electron flow in the connecting wires. Label this electron flow.
This diagram shows the electrolysis of copper chloride.
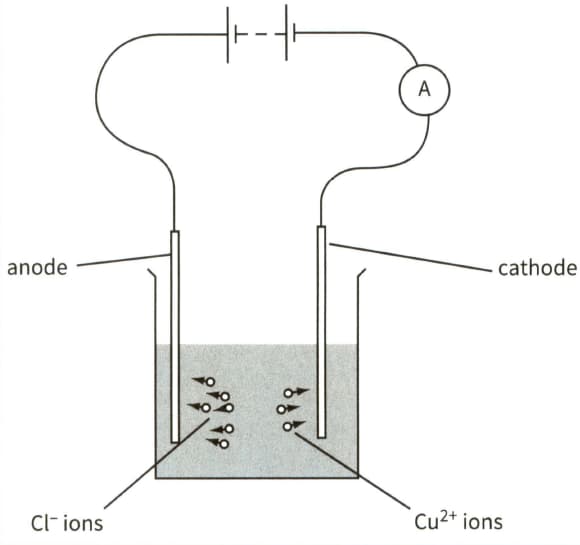
(b) In a time period of 8 minutes, chloride ions are neutralised and liberated at the anode and copper ions are neutralised and deposited on the cathode.
(i) Calculate the total charge passing through the electrolyte in this time.
This diagram shows the electrolysis of copper chloride.
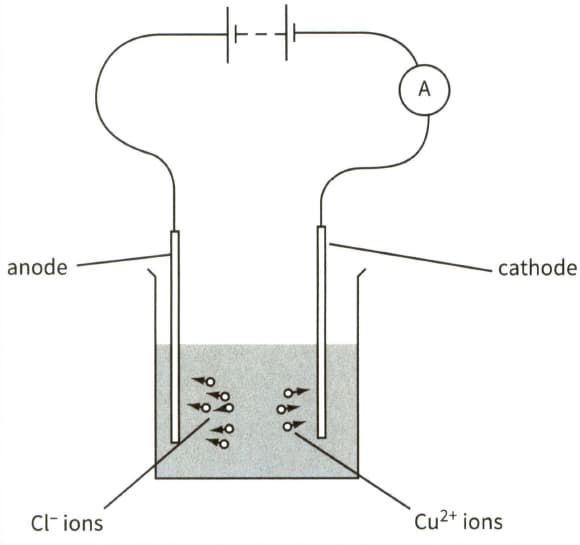
(b) In a time period of 8 minutes, chloride ions are neutralised and liberated at the anode and copper ions are neutralised and deposited on the cathode.
(ii) Calculate the current in the circuit.
This diagram shows an electron tube. Electrons moving from the cathode to the anode constitute a current. The current in the ammeter is .
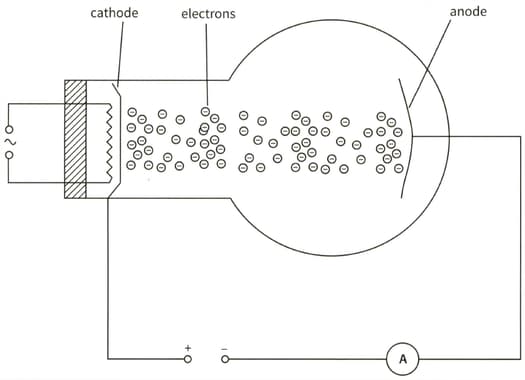
(a) Calculate the charge passing through the ammeter in 3 minutes.
This diagram shows an electron tube. Electrons moving from the cathode to the anode constitute a current. The current in the ammeter is .
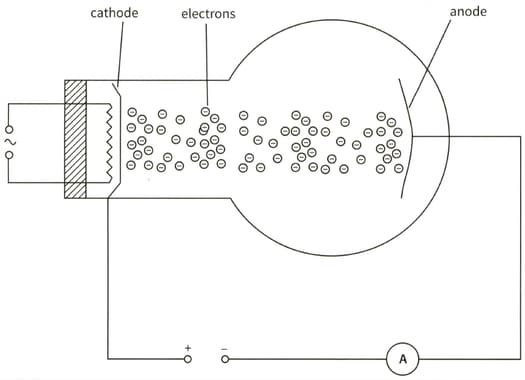
(b) Calculate the number of electrons that hit the anode in 3 minutes.
A length of copper track on a printed circuit board has a cross-sectional area of The current in the track is . You are provided with some useful information about copper:
of copper has a mass of
of copper contains atoms In copper, there is roughly one electron liberated from each copper atom
(b) Calculate the mean drift velocity of the electrons.
(a) Explain the difference between potential difference and e.m.f.
(b) A battery has negligible internal resistance, an e.m.f. of and a capacity of (ampere-hours). Calculate:
(i) The total charge that it can supply
