This question is about some metals and metal compounds. Hafnium, , forms a hydrated peroxide whose formula can be written as . Use the values below to calculate the relative molecular mass of hydrated hafnium peroxide. ( values: ).

Important Questions on Atoms, Molecules and Stoichiometry
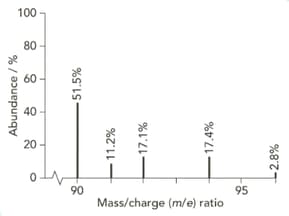
The mass spectrum of zirconium is shown below.
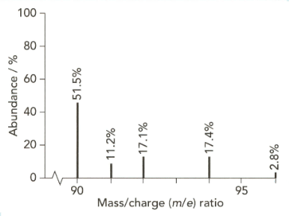
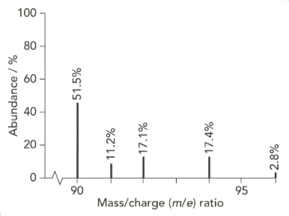
Give the isotopic symbol for the most abundant isotope of zirconium.
The mass spectrum of zirconium is shown below.
Use the information from this mass spectrum to calculate the relative atomic mass of zirconium. Give your answer to significant figures.
The mass spectrum of zirconium is shown below.
High-resolution mass spectra show accurate relative isotopic masses. State the meaning of the term relative isotopic mass?
A sample of of tin(IV) oxide is mixed with of carbon and heated. A reaction occurs.
Show by calculation that the reagent in excess is tin(IV) oxide. ( values: ).
A sample of zirconium is made by reacting of zirconium tetrachloride, , with excess magnesium.
The mass of zirconium produced is . Calculate the percentage yield of zirconium. ( values: ).
Solid sodium carbonate reacts with aqueous hydrochloric acid to form aqueous sodium chloride, carbon dioxide and water.
Rewrite this equation to include state symbols.