This question is about the ionic bond formed between the metal lithium (proton number ) and the non-metal fluorine (proton number ).
Draw a diagram to show what happens when a lithium atom reacts with a fluorine atom.
Important Questions on Atoms Combining
This question is about the ionic bond formed between the metal lithium (proton number ) and the non-metal fluorine (proton number ).
Write a word equation for the reaction between lithium and fluorine.
This diagram represents a molecule of a certain gas.
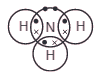
Name the gas, and give is formula.
This diagram represents a molecule of a certain gas.
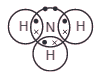
What do the symbols • and x symbols represent?
This diagram represents a molecule of a certain gas.
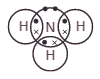
Which type of bonding holds the atoms together?
This diagram represents a molecule of ammonia.
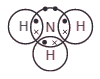
Name another compound with this type of bonding.
