EASY
Earn 100
Three closed rigid vessels, A, B and C without energy transfer with surroundings, which initially contain three different gases at different temperatures are connected by tube of negligible volume. The vessel A contains gas, at , vessel 'B' contains gas at and vessel 'C' contains gas at temperature . What is the final pressure (in atm) attained by gases when all valves of connecting three vessels are opened and additional heat supplied to vessel through valve. The volume of A, B and C vessel is respectively.
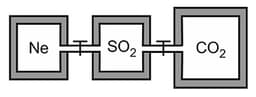
Given :
(a)
(b)
(c)
(d)None of these
50% studentsanswered this correctly
Important Questions on Thermodynamics
EASY
EASY
MEDIUM
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
EASY
MEDIUM
HARD
The specific heat of a certain substance is . Assuming ideal solution behavior, the energy required (in ) to heat of molal of its aqueous solution from to is closest to :
[Given: molar mass of the substance ; specific heat of water ]
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
of nitrous oxide gas is cooled at a constant pressure of atm from to causing the compression of the gas from to . The change in internal energy of the process, is . The value of is _____.
[nearest integer]
(Given: atomic mass of and of . Molar heat capacity of is )
EASY
EASY
EASY
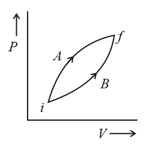
HARD

The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:

