Three moles of electrons are passed through three solutions in succession containing and , respectively. The molar ratio of amounts of cations reduced at cathode will be

Important Questions on Electrochemistry
The following reaction takes place at .
If an excess of tin metal is added to , what is the concentration of at equilibrium?
Answer correct up to one place of decimal.
Calculate the equilibrium constant for the reaction . The standard reduction potentials in acidic-medium conditions are and respectively for and couples.
If the answer is of type , report the value of correct up to the nearest integer.
In the following process of disproportionation,
Initial concentration of chlorate ion was . The equilibrium concentration of per chlorate ion will be . Hence is
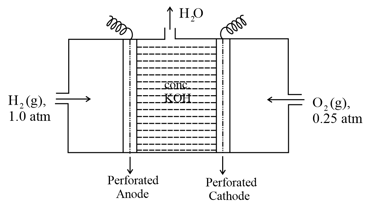
The efficiency of fuel cell is _____ percent(97.9/82.68/92.70).
Consider a efficient hydrogen - oxygen fuel cell working under standard conditions at bar and . Its cell reaction is
The work derived from the cell on the consumption of of is used to compress of a monoatomic ideal gas in a thermally insulated container. What is the change in the temperature (in ) of the ideal gas?
The standard reduction potentials for the two half - cells are given below
Use
