HARD
Earn 100
Three separate reversible processes are carried out with mole of an ideal gas initially at STP. In these processes, the pressure () is measured while increasing any one of the quantities volume (), temperature () and number of moles , keeping the other two constant. Plots of versus are found to be linear, where or or . For the processes and , the numerical values of the slopes and of the versus plots are found to follow the order , respectively. The correct statement(s) about these processes is/are
(a)work done is zero for process
(b)heat is completely converted into work for process
(c)entropy remains constant in process
(d)the average speed of molecules remains constant for process
50% studentsanswered this correctly
Important Questions on Thermodynamics
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
HARD
(: pressure, : volume, : temperature, : enthalpy, : entropy)
EASY
MEDIUM
EASY
Among the following the number of state variable is
Internal energy
Volume
Heat
Enthalpy
EASY
EASY
Observe the following properties:
Volume, enthalpy, density, temperature, heat capacity, pressure, internal energy.
The number of extensive properties in the above list is
EASY
Reversible process
MEDIUM
The total number of intensive properties from the following is _____.
Volume, Molar heat capacity, molarity, ,Gibbs free energy change, Molar mass, Mole
EASY
(Latent heat of ice is and )
HARD
One mole of an ideal monoatomic gas undergoes two reversible processes and as shown in the given figure:
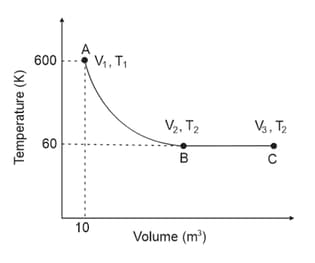
is an adiabatic process. If the total heat absorbed in the entire process and is , the value of is
[Use molar heat capacity of the gas at constant pressure, ]
EASY
EASY
EASY
i)
ii)
iii)
iv)

