EASY
Earn 100
Total energy of an isolated system can neither be created nor destroyed, although it can be transferred from one form to another. This is stated by:
(a)Zeroth law of thermodynamics
(b)First law of thermodynamics
(c)Second law of thermodynamics
(d)Third law of thermodynamics
50% studentsanswered this correctly
Important Questions on Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other

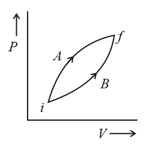
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
(R = 8.314 J/mol K) (ln7.5 = 2.01)

