EASY
Earn 100
Total number of and -bonds are in naphthalene is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
EASY
The number of hybridized carbon atoms in
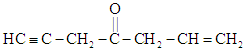
EASY
Why are most carbon compounds poor conductors of electricity?
MEDIUM
Wohler first prepared an organic compound from an inorganic compound in the laboratory. What is the organic compound?
MEDIUM
Identify the correct order of boiling points of the following compounds
(A)
(B)
(C)
EASY
In molecule, the hybridization of carbon and respectively, are :
MEDIUM
Which group of compounds shows correct increasing order of boiling points:
EASY
Which type of bond is formed by the carbon with other carbon atoms?
EASY
The correct order of catenation is:
EASY
Which of the following is an incorrect statement?
MEDIUM
The number of hybridised carbons in an acyclic neutral compound with molecular formula is
EASY
When water gas is mixed with half its volume of hydrogen and the mixture is compressed 300 atmospheric pressure and passed over catalyst a colourless liquid is obtained which is used as a solvent for paint and varnishes. The liquid will be
EASY
How many pi bonds and sigma bonds are present in following molecule?
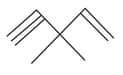
MEDIUM
Number of covalent bonds present in benzene is:
MEDIUM
Compare three properties of the organic and the inorganic compound.
MEDIUM
Which is the most stable?
EASY
The hydrocarbon with seven carbon atoms containing a neopentyl and a vinyl group is:
HARD
The functional group present in a molecule having the formula is
MEDIUM
Which of the following statements are usually correct for carbon compounds? These
(i) are good conductors of electricity
(ii) are poor conductors of electricity
(iii) have strong forces of attraction between their molecules
(iv) do not have strong forces of attraction between their molecules
MEDIUM
Which of the following structures contain -hybridised carbon atom(s)?
I.
II.
III. 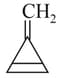
IV. 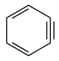
EASY
The number of C - C sigma bonds in the compound


