HARD
Earn 100
Total number of layers between a distance of in lattice (including first and last) are:
50% studentsanswered this correctly
Important Questions on Solid State
MEDIUM
MEDIUM
The edge length of a solid possessing cubic unit cell is (structure I), based on hard sphere model, which upon subjecting to a phase transition, a new cubic structure (structure II) having an edge length of is obtained, where is the radius of the hard sphere. Which of the following statements is true ?
MEDIUM
HARD
HARD
A two dimensional solid is made by alternating circles with radius and such that the sides of the circles touch. The packing fraction is defined as the ratio of the area under the circles to the area under the rectangle with sides of length and
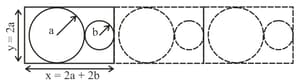
The ratio for which the packing fraction is minimized is closest to:
EASY
MEDIUM
EASY
MEDIUM
EASY
EASY

EASY
MEDIUM
EASY
converts from molten state to its solid state in structure. What is the number of the nearest atoms?
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM

