HARD
Earn 100
Tritium (an isotope of H) combine with fluorine to form a weak acid TF which ionises to give T+. Tritium is radioactive and a . A freshly prepared dilute aqueous solution of TF has a pT (equivalent of pH) of 1.7 and freezes at - 0.372. If 600 mL of freshly prepared solution were allowed to stand for 24.8 years, calculate ionisation constant of TF. Charge carried by emitted by tritium in faraday.
[Given : Kf for H2O = 1.86, t1/2 (T) = 12.4 yrs.]
(a)0.054 Faraday
(b)24.800 Faraday
(c)3.725 Faraday
(d)12.346 Faraday
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
HARD
EASY
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
In the radioactive decay process
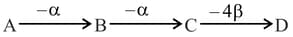
1. A and B are isobars
2. A and D are isotopes
3. C and D are isobars
4. A and C are isotopes
EASY
EASY
MEDIUM
In the decay sequence:
and are the particles/ radiation emitted by the respective isotopes. The correct option(s) is/are:
MEDIUM
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
HARD
HARD
HARD

