MEDIUM
10th CBSE
IMPORTANT
Earn 100
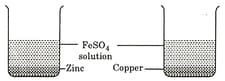
Two beakers A and B contain an aqueous solution of FeSO4. In the beaker A, zinc granules and in beaker B copper turnings have been placed. A grey coating was observed on zinc but not on copper. From the above observations, we can conclude.

(a)
zinc is more reactive than iron and copper.
(b)
iron is more reactive than zinc and copper.
(c)
iron is more reactive than zinc but less than copper.
(d)
copper is more reactive than iron but less than zinc.
100% studentsanswered this correctly

Important Questions on Metals and Non-Metals
HARD
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
HARD
10th CBSE
IMPORTANT
A student performed the following four experiments.
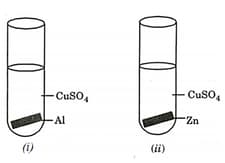
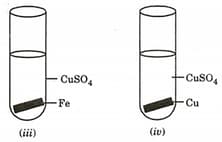
The experiments in which solid deposition on the metal plate will be observed are:
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
HARD
10th CBSE
IMPORTANT
HARD
10th CBSE
IMPORTANT
HARD
10th CBSE
IMPORTANT
