HARD
Earn 100
Two bulbs and , connected by a mercury manometer, are held in a thermostat, as shown below. The volume of is twice that of . contains as gas at the same pressure as the nitrogen in .
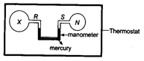
When the thermostat temperature is increased. Which of the following gases in bulb would cause the mercury level in the right hand limb of the manometer to rise?

(a)An equilibrium mixture
(b)An equilibrium mixture
(c)An equilibrium mixture
(d)An equilibrium mixture
50% studentsanswered this correctly
Important Questions on Kinetic Theory
HARD
If of gas A contains molecules, how many molecules of gas will be present in of ? The gases and are under the same conditions of temperature and pressure. Name the law on which the problem is based.
EASY
HARD
EASY
MEDIUM
EASY
“The volume of gas molecules is taken into consideration in Avogadro’s Law.”
HARD
Calculate the amount of each reactant required to produce of carbon dioxide, when two volumes of carbon monoxide combine with one volume of oxygen to produce two volumes of carbon dioxide.
State the law associated with this question.
EASY
EASY
MEDIUM
EASY
HARD
(Atmospheric pressure = of Hg)
MEDIUM
EASY
EASY
EASY
MEDIUM
( is universal gas constant and is the acceleration due to gravity)
MEDIUM
[Take gas constant as
EASY
EASY
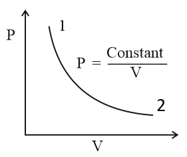
Out of the following which one correctly represents the diagram?

