HARD
11th CBSE
IMPORTANT
Earn 100
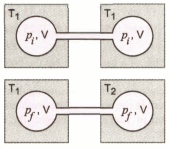
Two closed bulbs of equal volume () containing an ideal gas initially at pressure and temperature are connected through a narrow tube of negligible volume as shown in the figure below. The temperature of one of the bulbs is then raised to . The final pressure is

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
EASY
11th CBSE
IMPORTANT
EASY
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
