HARD
NEET
IMPORTANT
Earn 100
Two flasks of equal volume are connected by a narrow tube (of negligible volume) all at and contain moles of at . One of the flasks is then immersed into a bath kept at , while the other remains at . The number of moles of in flask and flask are:
(a)Moles in flask , Moles in flask
(b)Moles in flask , Moles in flask
(c)Moles in flask , Moles in flask
(d)Moles in flask , Moles in flask
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
At low pressures (For mole), the Van der Waal's equation is written as
.
The compressibility factor is then equal to:
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
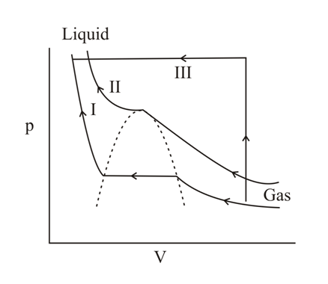
A gas can be condensed to liquid through the paths and , as shown in the figure. The path(s) through which the gas does not change to liquid abruptly is (are)