EASY
KVPY Aptitude Test - Stream SX
IMPORTANT
Earn 100
Two hydrogen atoms are in excited state with electrons residing in First one is moving towards left and emits a photon of energy towards right. Second one is moving towards right with same speed and emits a photon of energy towards right. Taking recoil of nucleus into account during emission process
(a)
(b)
(c)
(d)information insufficient
100% studentsanswered this correctly

Important Questions on Modern Physics - I
EASY
KVPY Aptitude Test - Stream SX
IMPORTANT
In a hydrogen atom following the Bohr's postulates the product of linear momentum and angular momentum is proportional to where is the orbit number. Then is:
EASY
KVPY Aptitude Test - Stream SX
IMPORTANT
The wavelengths of rays of two metals and are and respectively, where is Rydberg's constant. The number of elements lying between and according to their atomic numbers is
EASY
KVPY Aptitude Test - Stream SX
IMPORTANT
One of the lines in the emission spectrum of has the same wavelength as that of the line of Balmer series in hydrogen spectrum. The electronic transition corresponding to this line is:
EASY
KVPY Aptitude Test - Stream SX
IMPORTANT
If the short wavelength limit of the continuous spectrum coming out of a Coolidge tube is then the de-Broglie wavelength of the electrons reaching the target metal in the Coolidge tube is approximately:
EASY
KVPY Aptitude Test - Stream SX
IMPORTANT
The photon radiated from hydrogen corresponding to line of Lyman series is absorbed by a hydrogen like atom in excited state. As a result the hydrogen like atom makes a transition to orbit. Then,
EASY
KVPY Aptitude Test - Stream SX
IMPORTANT
In a photoelectric experiment, with light of wavelength the fastest electron has speed If the exciting wavelength is changed to the speed of the fastest emitted electron will become
EASY
KVPY Aptitude Test - Stream SX
IMPORTANT
The element which has a rays line of wavelength is and
EASY
KVPY Aptitude Test - Stream SX
IMPORTANT
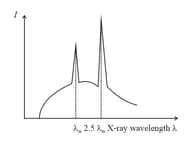
When an electron accelerated by potential difference is bombarded on a specific metal, the emitted ray spectrum obtained is shown in adjoining graph. If the potential difference is reduced to the correct spectrum is