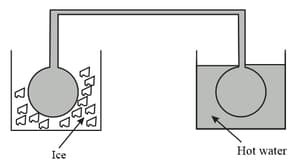
Two identical glass bulbs are interconnected by a thin glass tube at . A gas is filled in these bulbs. If one bulb is placed in ice and another bulb is placed in hot bath, the pressure of the gas become times. The temperature of hot bath will be.


Important Questions on Kinetic Theory of Gases
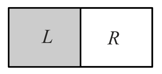
A vessel is partitioned in two equal halves by a fixed diathermic separator. Two different ideal gases are filled in left and right halves. The root-mean-square speed of the molecules in part is equal to the mean speed of molecules in the part. Then the ratio of the mass of a molecule in part to that of a molecule in part is:-
Read the given statements and decide which is/are correct on the basis of kinetic theory of gases
(I) Energy of one molecule at absolute temperature is zero.
(II) The rms speeds of different gases are same at same temperature.
(III) For one gram of all ideal gases kinetic energy is same at same temperature.
(IV) For one mole of all ideal gases' mean kinetic energy is same at same temperature.
