EASY
NEET
IMPORTANT
Earn 100
Two identical masses of each fall on a wheel from a height of . The wheel disturbs a mass of water. The rise in temperature of water will be,
50% studentsanswered this correctly
Important Questions on Thermal Properties of Matter
EASY
NEET
IMPORTANT
A block of mass is heated to a temperature of and placed on a large ice block. What is the maximum amount of ice that can melt (approximately)?
(specific heat for the body)
MEDIUM
NEET
IMPORTANT
of ice at is mixed with of water at . The final temperature of mixture is,
(specific heat of ice, specific heat of water , Latent heat of ice )
MEDIUM
NEET
IMPORTANT
The amount of heat required to convert of ice at into steam at is,
Specific heat of water , Latent heat of vaporisation
EASY
NEET
IMPORTANT
The latent heat of vaporisation for water is . Its value in joule/kg will be,
MEDIUM
NEET
IMPORTANT
If ice at is mixed with water at , the final temperature will be,
EASY
NEET
IMPORTANT
of energy supplied to of water will raise its temperature by nearly,
MEDIUM
NEET
IMPORTANT
The graph shown in the figure represents the change in temperature of of a substance as it absorbs heat at a constant rate of . The latent heat of vaporization of the substance is,
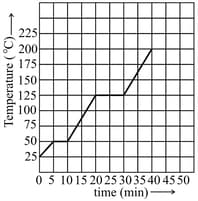
EASY
NEET
IMPORTANT
A block of ice with mass falls into a lake. After impact, a mass of ice melts. Both the block of ice and the lake have a temperature of . If represents the heat of fusion, the minimum distance the ice fell before striking the surface is,
