MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Two inorganic compounds and were heated in a dry test tube. evolved a colourless gas which turned lead acetate paper black and evolved a gas which turned lime water milky. The anions in and B respectively are:
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Qualitative Analysis
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
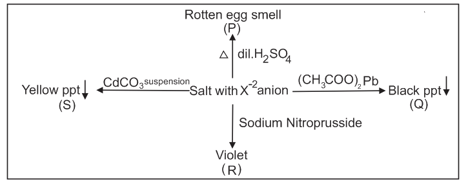
Anion is:
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
