MEDIUM
Chemistry
IMPORTANT
Earn 100
Two liquids and have at a certain temperature. If the mole fraction ratio of , the mole fraction of in vapour in equilibrium with the solution at a given temperature is -
(a)0.1
(b)0.2
(c)0.5
(d)1.0
66.67% studentsanswered this correctly

Important Questions on Solutions
EASY
Chemistry
IMPORTANT
Melting point, m.p Freezing point depression constant,
HARD
Chemistry
IMPORTANT
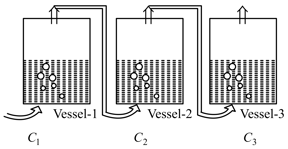
EASY
Chemistry
IMPORTANT
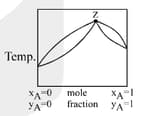
MEDIUM
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
moles of is heated to form and . As soon as and are formed they react to form .
are simultaneously established. At equilibrium, the degree of dissociation of was found to be . Which of the following is/are incorrect at equilibrium?
MEDIUM
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
