HARD
Earn 100
Two metal P and Q belong to the second group of periodic table. P forms insoluble oxide but soluble sulphate Q forms a soluble oxide but insoluble sulphate. Hydroxide of metal P is soluble in NaOH while that of metal Q is insoluble in NaOH. What are metal P and Q?
(a)P = Be, Q = Ba
(b)P = Mg, Q = Ca
(c)P = Ca, Q = Sr
(d)P = Ba, Q = Mg
50% studentsanswered this correctly
Important Questions on The s-Block Elements
EASY
MEDIUM
Reaction of with gives :
(A)
(B)
(C)
(D)
(E)
Choose the correct answer from options given below
EASY
EASY
EASY
EASY
MEDIUM
HARD
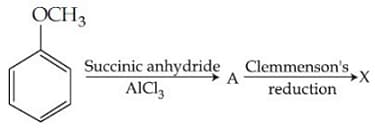
X is :
MEDIUM
HARD
EASY
MEDIUM
EASY
EASY
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM

