Two reactions, (i) Products, (ii) Products, follow first order kinetics. The rate of reaction (i) is doubled when the temperature is raised from to . The half- life of this reaction at is . At the same temperature, decomposes twice as fast as . Calculate the energy of activation for reaction (i) and calculate the rate constant of reaction (ii), at .

Important Questions on Chemical Kinetics
The experimental data for the reaction : is :
| Experiment |
Initial rate |
||
Write the most probable rate equation for the reaction giving reason for your answer.
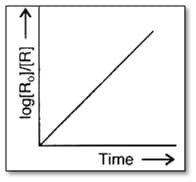
Answer the following question on the basis of the curve given below for a zero order reaction :
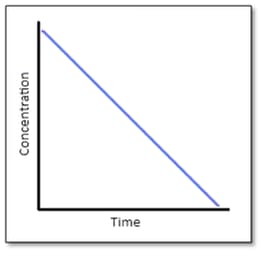
Find the relation between slope of this line and rate constant.
Answer the following question on the basis of the curve given below for a zero order reaction :
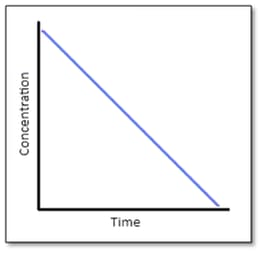
If the slope is , then, calculate the rate constant.
Consider the reaction, . The change in concentration with time is shown in the graph. Predict the order of the reaction.
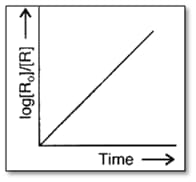
Consider the reaction, . The change in concentration with time is shown in the graph. Derive the expression for the time which is
required to complete the reaction.
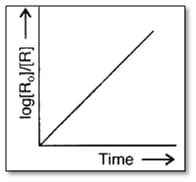
Consider the reaction, . The change in concentration with time is shown in the graph. Predict the order of the reaction.
changes to by successive radioactive decay. A sample of uranium was analyzed and found to contain of and of had accumulated due to decay of, find out the age of ore. (Half-life of years).
For the hydrolysis of methyl acetate in the aqueous solution, following results were obtained :
Show that it follows pseudo first order reaction, as the concentration of water remains constant.
