HARD
Earn 100
Two reactions (I) Products (II) Products follow first order kinetics. The rate of the reaction (I) is doubled when temperature is raised from to . The half-life for this reaction at is minute. At the same temperature decomposes twice as fast as . If the energy of activation for the reaction (II) is half that of reaction (I) calculate the rate constant of reaction (II) at
Important Questions on Chemical Kinetics
EASY
HARD
| Experiment No. | Rate of reaction | |||
The rate of the reaction for and is found to be The value of is __________
MEDIUM
In the following reaction;
‘A’ and ‘B’ respectively can be:
HARD
The results given in the below table were obtained during kinetic studies of the following reaction:
| Experiment | Initial rate/ | ||
| I | |||
| II | |||
| III | |||
| IV | X | ||
| V | Y |
X and Y in the given table are respectively :
MEDIUM
(i)
(ii)
(iii)
The overall order of the reaction will be
MEDIUM
EASY
The initial concentration of is and it is after 30 minutes. The rate of formation of is:
MEDIUM
EASY
If the concentration of is increased from , keeping the value of at , the rate constant will be:
HARD
...(i)
...(ii)
The closest rate constant for the overall reaction
is:
EASY
Consider the following reactions
The order of the above reactions are respectively. The following graph is obtained when log[rate] vs.log[conc.] are plotted:
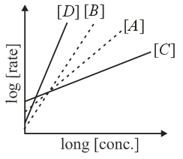
Among the following, the correct sequence for the order of the reactions is :
EASY
Rate
If the concentration of A is kept the same but that of B is doubled what will happen to the rate itself?
MEDIUM
MEDIUM
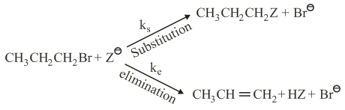
where,
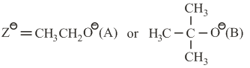
and , are respectively, the rate constants for substitution and elimination, and , the correct option is ________
EASY
Which of the following expression is correct for the rate of reaction given below ?
EASY
MEDIUM
EASY
MEDIUM
HARD
For an elementary chemical reaction, , the expression for is:

