Ultraviolet light with photons of energy is shone onto a zinc plate. The work function of zinc is . Calculate the maximum energy with which an electron can be emitted from the zinc plate. Give your answer in J.

Important Questions on Quantum Physics
The diagram shows five of the energy levels in a helium ion. The lowest energy level is known as the ground state.
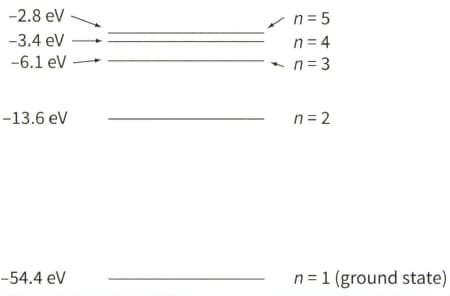
Determine the energy, in joules, that is required to completely remove the remaining electron, originally in its ground state, from the helium ion.
The diagram shows five of the energy levels in a helium ion. The lowest energy level is known as the ground state.
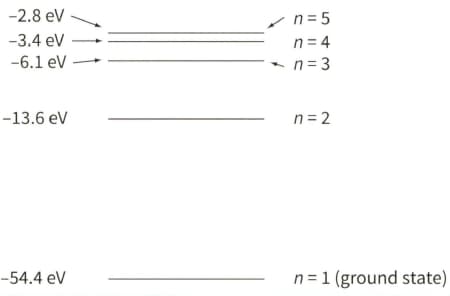
Determine the frequency of the radiation that is emitted when the electron drops from the level $n=3$ to $n=2$. State the region of the electromagnetic spectrum in which this radiation lies.
The diagram shows five of the energy levels in a helium ion. The lowest energy level is known as the ground state.
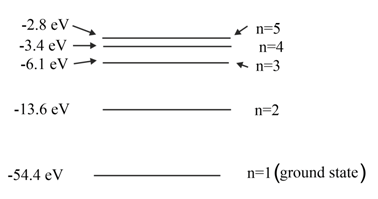
Without further calculation, describe qualitatively how the frequency of the radiation emitted when the electron drops from the level $n=2$ to $n=1$ compares with the energy of the radiation emitted when it drops from $n=3$ to $n=2$.
The spectrum of sunlight has dark lines. These dark lines are due to the absorption of certain wavelengths by the cooler gases in the atmosphere of the Sun.
One particular dark spectral line has a wavelength of $590 \mathrm{~nm}$. Calculate the energy of a photon with this wavelength. ()
The spectrum of sunlight has dark lines. These dark lines are due to the absorption of certain wavelengths by the cooler gases in the atmosphere of the Sun.
The diagram shows some energy levels of an isolated atom of helium.
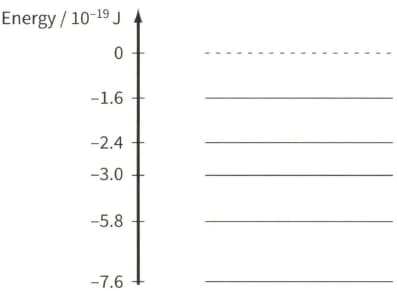
Explain the significance of the energy levels having negative values.
The spectrum of sunlight has dark lines. These dark lines are due to the absorption of certain wavelengths by the cooler gases in the atmosphere of the Sun.
The diagram shows some energy levels of an isolated atom of helium.
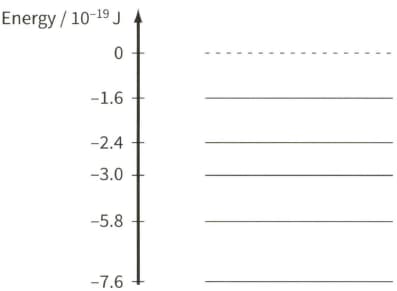
Explain, with reference to the energy level diagram, how a dark line in the spectrum may be due to the presence of helium in the atmosphere of the Sun.
The spectrum of sunlight has dark lines. These dark lines are due to the absorption of certain wavelengths by the cooler gases in the atmosphere of the Sun.
The diagram shows some of the energy levels of an isolated atom of helium.
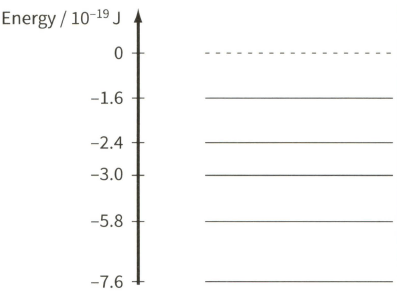
All the light absorbed by the atoms in the Sun's atmosphere is re-emitted. Suggest why a dark spectral line of wavelength of $590 \mathrm{~nm}$ is still observed from the Earth.
