EASY
Earn 100
Under critical conditions, the compressibility factor for a gas is -
(a)
(b)
(c)1
(d)
50% studentsanswered this correctly
Important Questions on States of Matter
HARD
MEDIUM
MEDIUM

HARD

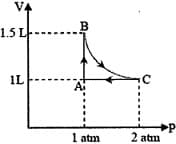
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
EASY
Given below are two statement : one is labelled as Assertion and the other is labelled as Reason .
Assertion is adsorbed to a large extent than on activated charcoal.
Reason : has a higher critical temperature than
In the light of the above statements, choose the most appropriate answer from the options given below.
HARD

The correct option(s) is (are)
EASY
(Latent heat of ice is and )
EASY
MEDIUM
EASY
Among the following gases, the order of liquefiability is
a)
b)
c)
d)
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
EASY
MEDIUM
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is




EASY
EASY
MEDIUM
[Heat of fusion of ice ; Specific heat of water ]
EASY
Which of the following gases can be absorbed in more proportion?
MEDIUM
MEDIUM
| Gas | ||||
|---|---|---|---|---|
Which gas is expected to have the highest critical temperature?

