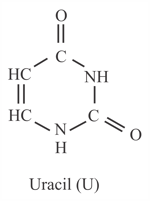
Uracil is base present in with the following structure. in uracil is _______.


Important Questions on Some Basic Concepts of Chemistry
The number of units, which are used to express concentration of solutions from the following is _________.
(Mass percent, Mole, Mole fraction, Molarity, ppm, Molality.)
In sulphur estimation. of an organic compound gave of barium sulphate. The percentage of sulphur in the compound is _______(Nearest Integer)
(Given: Atomic mass Ba: )
The volume of , containing , required to completely neutralise obtained by reacting of metallic sodium with water, is _____ . (Nearest Integer)
(Given : molar Masses of are and respectively)
Some amount of dichloromethane is added to of chloroform to prepare solution of . The concentration of is _____ ppm (by mass).
Given: Atomic mass : density of
